The diagnostic trouble code P03DF indicates an issue with the cylinder 8 pressure sensor circuit on the vehicle's engine. This code sets when the powertrain control module (PCM) detects an intermittent or erratic signal from the cylinder 8 pressure sensor.
Car Exhaust and Acid Rain: The Damaging Effects and Mitigation Strategies
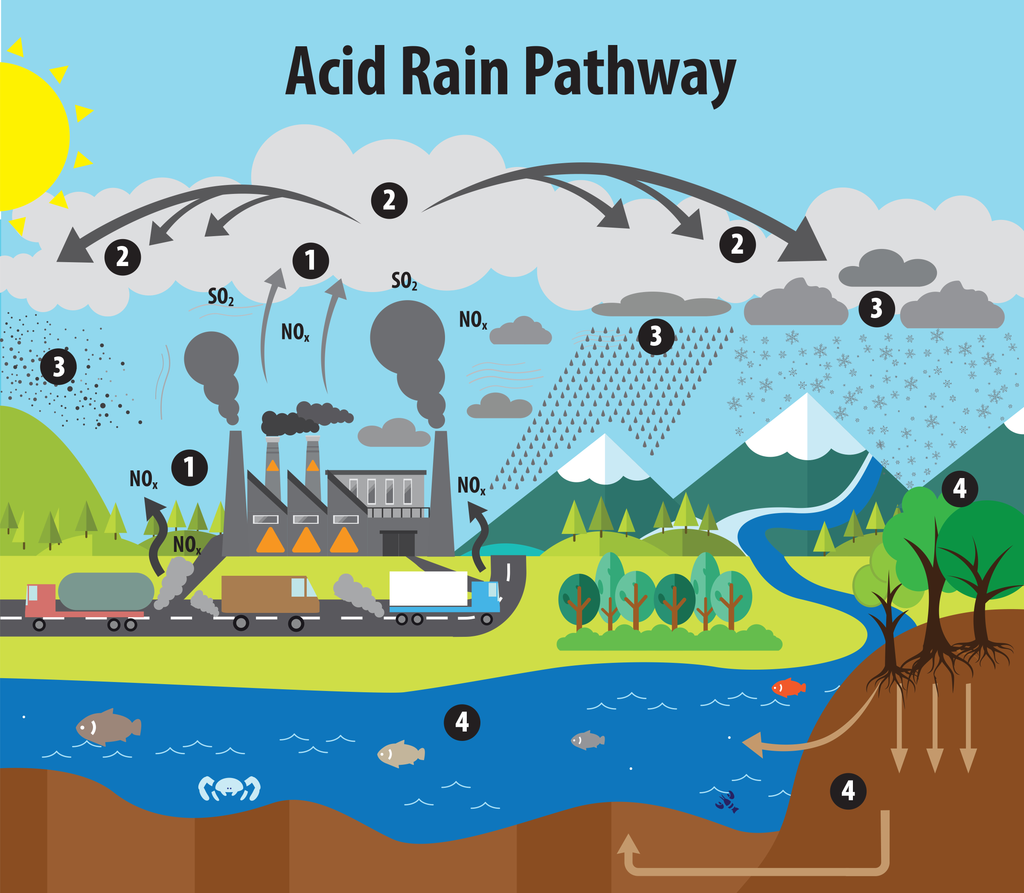
Car Exhaust and Acid Rain: The Damaging Effects and Mitigation Strategies
The impact of car exhaust emissions on acid rain formation is a pressing environmental concern with far-reaching consequences. This article delves into the alarming effects of vehicular pollution on the acidification of precipitation and explores effective strategies to mitigate this global issue. We will examine the chemical processes involved, the detrimental impacts on ecosystems and human health, and the multi-faceted approach required to address this challenge effectively.

I. The Alarming Impact of Car Exhaust on Acid Rain Formation
Acid rain, a form of precipitation with high levels of acidity, is primarily caused by the emission of certain pollutants from human activities, particularly the burning of fossil fuels in vehicle engines. This phenomenon has severe implications for the environment and human well-being. Understanding the chemical processes behind acid rain formation is crucial to addressing this issue effectively.
A. The Chemical Process Behind Acid Rain
When fossil fuels are burned in vehicle engines, they release nitrogen oxides (NOx) and sulfur dioxide (SO2) into the atmosphere. These pollutants undergo a series of chemical reactions with water molecules, oxygen, and other compounds present in the air, as shown in the table below:
| Pollutant | Chemical Reaction | Acid Formed |
|---|---|---|
| Nitrogen Oxides (NOx) | Reaction with water molecules | Nitric Acid (HNO3) |
| Sulfur Dioxide (SO2) | Reaction with water and oxygen | Sulfuric Acid (H2SO4) |
These acids then dissolve in water droplets, forming acidic precipitation that falls to the ground as acid rain, snow, or fog. The acidity levels of precipitation can vary depending on the concentration of these acids, with lower pH values indicating higher acidity.
B. The Damaging Environmental Consequences
Acid rain has a profound impact on both aquatic and terrestrial ecosystems, causing significant damage to various components of the environment.
Aquatic Ecosystems: When acid rain falls into lakes, rivers, and streams, it lowers the pH levels of the water, making it more acidic. This increased acidity can be detrimental to aquatic life, causing harm to fish, amphibians, and other organisms that rely on these water bodies. Some species may struggle to reproduce or even face extinction due to the acidification of their habitats.
Soil and Vegetation: Acid rain leaches essential nutrients from the soil, making it less fertile and hindering plant growth. This nutrient depletion can lead to soil erosion and damage to terrestrial vegetation, affecting entire ecosystems and disrupting the food chain.
Infrastructure Damage: Acid rain accelerates the weathering and corrosion of buildings, monuments, and infrastructure made of limestone, marble, and other materials susceptible to acid corrosion. This can result in significant economic costs for repair and maintenance, as well as the potential loss of cultural heritage sites.
The environmental consequences of acid rain are far-reaching and can have long-lasting impacts on ecosystems, biodiversity, and the overall health of our planet.
II. The Detrimental Effects on Human Health
Acid rain not only affects the environment but also poses significant risks to human health. The pollutants that contribute to acid rain formation, such as nitrogen oxides and sulfur dioxide, can have direct impacts on respiratory systems and cardiovascular health.
A. Respiratory Problems and Asthma Exacerbation
Exposure to these pollutants can cause respiratory problems, exacerbate existing conditions like asthma, and increase the risk of developing lung diseases. Individuals with pre-existing respiratory conditions are particularly vulnerable to the effects of acid rain-causing pollutants. The table below summarizes some of the respiratory health impacts associated with acid rain:
| Pollutant | Respiratory Health Effects |
|---|---|
| Nitrogen Oxides (NOx) | - Lung inflammation - Reduced lung function - Increased asthma symptoms |
| Sulfur Dioxide (SO2) | - Bronchial constriction - Aggravated asthma attacks - Increased respiratory infections |
B. Cardiovascular Disease Risks
Studies have also linked acid rain and its associated pollutants to an increased risk of cardiovascular diseases. Long-term exposure to these pollutants can contribute to the development of heart and circulatory system problems, such as:
Increased risk of heart attacks
Elevated blood pressure
Arterial damage and atherosclerosis
C. Public Health Concerns and Awareness
The potential health impacts of acid rain have raised significant public health concerns. Raising awareness about the risks associated with acid rain and promoting environmentally conscious choices is crucial in mitigating its effects on human well-being. Public education campaigns, healthcare initiatives, and policy changes can play a vital role in addressing this issue.
III. Mitigating Acid Rain: A Multi-Faceted Approach
Addressing the issue of acid rain requires a comprehensive and multi-faceted approach that involves various strategies and stakeholders. These strategies can be categorized into three main areas:
Emission Reduction Strategies
Sustainable Transportation Solutions
Transitioning to Renewable Energy Sources
A. Emission Reduction Strategies
One of the key strategies is reducing the emission of acid rain-causing pollutants from vehicles. This can be achieved through the implementation of emission control technologies and stricter emission standards and regulations.
Catalytic Converters and Emission Control Technologies
Modern vehicles are equipped with catalytic converters, which help convert harmful gases like nitrogen oxides and carbon monoxide into less harmful compounds, reducing their emission levels. Continuous advancements in emission control technologies play a crucial role in mitigating acid rain formation.
Some of the key emission control technologies include:
Catalytic converters
Exhaust gas recirculation (EGR) systems
Selective catalytic reduction (SCR) systems
Diesel particulate filters (DPFs)
Stricter Emission Standards and Regulations
Governments and international organizations have implemented stricter emission standards and regulations, requiring automakers to develop cleaner and more efficient engines. These regulations aim to limit the emission of pollutants from vehicles, thereby reducing the potential for acid rain formation.
Examples of emission standards and regulations include:
Euro 6 emission standards (European Union)
Tier 3 emission standards (United States)
Bharat Stage VI emission norms (India)
B. Sustainable Transportation Solutions
Promoting sustainable transportation alternatives is another essential strategy in mitigating acid rain. By reducing the number of vehicles on the road and their associated emissions, we can significantly contribute to a cleaner environment.
Promoting Public Transit and Cycling: Encouraging the use of public transportation, such as buses and trains, as well as cycling, can significantly reduce the number of individual vehicles on the road and their associated emissions. These modes of transportation not only reduce air pollution but also promote a healthier lifestyle and contribute to urban sustainability.
Adoption of Electric Vehicles: The adoption of electric vehicles, which produce zero direct emissions, is a promising solution for mitigating acid rain formation. As the technology continues to advance and become more accessible, the widespread adoption of electric vehicles can significantly reduce the reliance on fossil fuel-powered vehicles and their associated emissions.
C. Transitioning to Renewable Energy Sources
While addressing vehicle emissions is crucial, a broader approach is necessary to tackle the issue of acid rain effectively. Transitioning to renewable energy sources, such as solar, wind, and hydroelectric power, can significantly reduce the reliance on fossil fuels and the associated emissions from various sectors, including transportation and power generation.
Reducing Reliance on Fossil Fuels: By investing in clean and sustainable energy sources, we can reduce the burning of fossil fuels, which is a major contributor to acid rain formation. This transition not only mitigates acid rain but also addresses other environmental concerns, such as climate change and air pollution.
Long-term Strategies for Energy Transition: The transition to renewable energy sources is a long-term process that requires significant investments, policy changes, and technological advancements. Governments, industries, and individuals must work together to develop and implement long-term strategies for this energy transition, ensuring a sustainable future for generations to come.
Some examples of renewable energy sources include:
Solar power (photovoltaic and concentrated solar power)
Wind power (onshore and offshore wind farms)
Hydroelectric power (dams and run-of-river systems)
Geothermal energy
Bioenergy (biomass, biogas, and biofuels)
IV. International Cooperation and Collaborative Efforts

Acid rain is a global problem that transcends national boundaries. Addressing this issue requires international cooperation and collaboration among countries, organizations, and stakeholders.
A. Knowledge Sharing and Best Practices
Sharing knowledge and best practices among nations is crucial for developing and implementing effective solutions to mitigate acid rain. By collaborating and learning from each other's experiences, countries can adopt and adapt successful strategies to their local contexts.
Some examples of knowledge-sharing platforms and initiatives include:
International conferences and workshops
Research collaborations and joint studies
Online databases and knowledge repositories
Capacity-building programs and technical assistance
B. Transboundary Air Pollution and Global Cooperation
Acid rain is often a result of transboundary air pollution, where pollutants emitted in one country can contribute to acid rain formation in neighboring regions or even across continents. Global cooperation and coordinated efforts are essential to address this cross-border issue effectively.
Examples of international agreements and initiatives related to transboundary air pollution include:
Convention on Long-range Transboundary Air Pollution (CLRTAP)
Regional Air Pollution Information and Simulation (RAINS) program
Acid Deposition Monitoring Network in East Asia (EANET)
C. Regulations and Policies for Emission Reduction
International organizations and agreements play a vital role in establishing regulations and policies aimed at reducing emissions from various sectors, including transportation, industry, and power generation. These collective efforts help create a level playing field and ensure that all nations are working towards a common goal of mitigating acid rain and its associated environmental and health impacts.
Examples of international regulations and policies include:
Paris Agreement on Climate Change
International Maritime Organization (IMO) regulations on ship emissions
International Civil Aviation Organization (ICAO) standards for aircraft emissions
Addressing the issue of acid rain requires a concerted effort from individuals, communities, governments, and international organizations. By implementing emission reduction strategies, promoting sustainable transportation solutions, transitioning to renewable energy sources, and fostering international cooperation, we can mitigate the damaging effects of car exhaust on acid rain formation and create a healthier and more sustainable future for our planet.
FAQs
What are the main causes of acid rain?
The main causes of acid rain are emissions of sulfur dioxide and nitrogen oxides from burning fossil fuels, industrial processes, and vehicle exhaust. Natural causes like volcanic eruptions also contribute to a lesser extent.
How does acid rain form chemically?
Sulfur dioxide and nitrogen oxides react with water molecules in the atmosphere to form sulfuric acid and nitric acid respectively, which then get deposited as acid rain.
What are the effects of acid rain on aquatic life?
Acid rain increases the acidity of lakes and rivers, making the water toxic for many aquatic organisms. It also allows leaching of aluminum from soil into water bodies, further harming aquatic life.
How does acid rain impact forests and vegetation?
Acid rain leaches away nutrients like calcium and magnesium from soils, damaging plant life. It also causes aluminum to be released into soils, which is toxic for trees and other vegetation.
What are the effects of acid rain on buildings and infrastructure?
Acid rain accelerates the corrosion and weathering of buildings, statues, and other structures made of limestone, marble, and metals, leading to significant damage over time.
How does acid rain affect human health?
The gases that cause acid rain, like sulfur dioxide and nitrogen oxides, can lead to respiratory issues in humans. Acid rain also increases exposure to toxic metals like mercury and lead.
What is being done to reduce acid rain?
Governments have implemented regulations to limit emissions of sulfur dioxide and nitrogen oxides from power plants, industries, and vehicles, which has helped reduce acid rain in some regions.
How far can acid rain travel?
Acid rain can travel hundreds of miles from its source, carried by wind and air currents, affecting areas far away from the original emission sources.
What is the difference between wet and dry deposition of acid rain?
Wet deposition refers to acidic precipitation like rain, snow, or fog, while dry deposition involves acidic particles and gases settling directly on surfaces.
How does acid rain affect soil chemistry?
Acid rain can leach away essential nutrients from soils and increase the mobility of toxic metals like aluminum, leading to soil degradation and affecting plant growth.
Bình luận (0)
Bài viết đề xuất
Greetings, fellow gearheads! As a seasoned auto mechanic, I've encountered my fair share of diagnostic trouble codes, and the P0389 is one that tends to leave many car owners scratching their heads in bewilderment. But fear not, for today I'm going to delve deep into the intricacies of this code, providing you with a comprehensive understanding that will leave you feeling like a true automotive expert.
The Diagnostic Trouble Code (DTC) P03E8 refers to an issue with the "A Camshaft Position Actuator Position Sensor C Circuit High Bank 1." This code is part of the VAG (Volkswagen Auto Group) diagnostic system, which is used to identify and troubleshoot issues in vehicles manufactured by Volkswagen, Audi, SEAT, and Skoda, among others.
P03D8 is an OBD-II trouble code that indicates the engine control module (ECM) has detected low pressure variation in cylinder 7 of the engine. This code is specific to vehicles with cylinder pressure sensors that monitor the combustion pressure in each cylinder.
Bài viết liên quan
The cacophony of roaring engines and thunderous exhaust notes has become an unwelcome symphony in our urban landscapes. Noise pollution from automotive exhaust systems is a pervasive environmental issue that poses significant risks to human health and ecological well-being.
Vehicle emissions are a significant contributor to air pollution, releasing a complex mixture of harmful pollutants into the atmosphere. Among these pollutants are particulate matter from exhaust and non-exhaust sources, as well as ozone precursors like nitrogen oxides and volatile organic compounds





