The alternator plays a crucial role in a vehicle's electrical system by charging the battery and powering various components. However, issues with the voltage regulator, which controls the alternator's output, can lead to charging problems. In such cases, bypassing the voltage regulator can serve as a temporary solution to diagnose and troubleshoot the issue.
What Are Electric Vehicle Batteries Made Of? A Comprehensive Guide
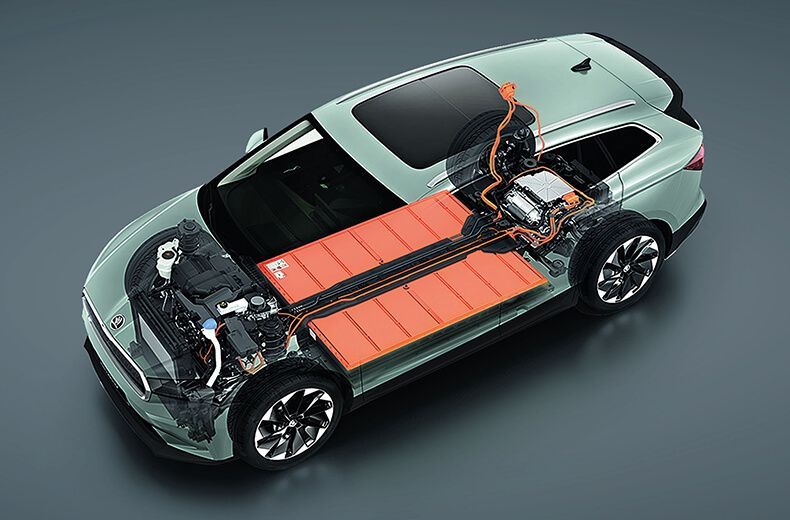
What Are Electric Vehicle Batteries Made Of? A Comprehensive Guide
Electric vehicles (EVs) have emerged as a sustainable alternative to traditional gasoline-powered cars, offering a solution to reduce emissions and mitigate the environmental impact of transportation. At the heart of these innovative vehicles lies a crucial component – the battery pack. This comprehensive guide delves into the intricate world of EV batteries, exploring their composition, manufacturing processes, different chemistries, and the ongoing efforts to enhance their sustainability and performance.

Introduction to Electric Vehicle Batteries
Electric vehicle batteries are complex systems designed to store and provide the energy required for propulsion. These batteries are primarily composed of lithium-ion (Li-ion) cells, which contain several critical minerals and materials. The most essential components are listed in the following table:
| Component | Description |
|---|---|
| Lithium | A highly reactive alkali metal that provides high energy density and enables efficient energy storage and release. |
| Cobalt | Enhances the battery's thermal stability and energy density, although its mining raises environmental and ethical concerns. |
| Graphite | Used in the anode, graphite facilitates the flow of lithium ions during charging and discharging. |
| Nickel | Used in the cathode to improve energy density and power output. |
| Manganese | Used in the cathode to improve energy density and power output. |
Lithium-Ion Battery Cells
Lithium-ion battery cells are the fundamental building blocks of EV batteries. Each cell consists of a cathode (positive electrode), an anode (negative electrode), an electrolyte solution that allows the flow of lithium ions, and a separator that prevents short circuits. The cathode is typically made of lithium nickel manganese cobalt oxide, while the anode is composed of graphite.
Battery Pack Construction
The manufacturing process of EV batteries involves several intricate steps:
The electrode materials are mixed with solvents, binders, and additives to create a slurry.
This slurry is then coated onto metal foils (aluminum for the cathode, copper for the anode) and dried.
The coated electrodes are compressed through a calendering process to increase energy density and uniformity.
The electrodes are cut into the desired shapes and assembled into individual battery cells.
These cells are then combined into modules, which are further integrated into the complete battery pack.
The pack is equipped with various electrical connections, monitoring systems, and a protective housing.
Battery Chemistries and Types
While Li-ion batteries dominate the EV market, several different chemistries are used, each with its own advantages and trade-offs. The table below summarizes the most common battery chemistries and their characteristics:
| Battery Chemistry | Cathode Material | Advantages | Disadvantages |
|---|---|---|---|
| Lithium Nickel Manganese Cobalt Oxide (NMC) | Nickel, manganese, and cobalt oxides | High energy density, good thermal stability | - |
| Lithium Iron Phosphate (LFP) | Lithium iron phosphate | Exceptional thermal stability, safe | Lower energy density |
| Lithium Nickel Cobalt Aluminum Oxide (NCA) | Nickel, cobalt, and aluminum oxides | High energy density | Concerns about thermal stability and cobalt supply |
Additionally, researchers are exploring emerging technologies like solid-state batteries and silicon anodes, which could potentially offer higher energy densities and improved safety.
Solid-state batteries replace the liquid electrolyte with a solid electrolyte, reducing the risk of thermal runaway and increasing energy density.
Silicon anodes have a higher capacity than graphite anodes, enabling higher energy densities.
Environmental Impact and Sustainability
While EVs have significantly lower emissions during operation compared to gasoline vehicles, the production of their batteries carries environmental concerns.
Mining and Mineral Extraction
The mining of critical minerals like lithium and cobalt can lead to:
Water pollution
Habitat destruction
Human rights issues
Responsible mining practices and sustainable sourcing of materials are crucial to mitigate these impacts.
Manufacturing Process
The manufacturing process of EV batteries requires substantial energy and water resources. Transitioning to renewable energy sources for battery manufacturing and charging can significantly reduce the overall carbon footprint of EVs.
Battery Recycling and Second-Life Applications
To address the environmental impact of battery production, it is essential to prioritize battery recycling and second-life applications. The benefits of these practices include:
Extending the lifespan of batteries
Reducing the demand for new battery production
Promoting a circular economy
Repurposing retired EV batteries for energy storage systems or other applications can contribute to these benefits.
Future Trends and Innovations
The EV industry is continuously striving to improve battery performance, safety, and sustainability. Key areas of focus include:

Higher Energy Densities
Ongoing research efforts focus on developing batteries with higher energy densities, enabling longer driving ranges and improved performance for electric vehicles.
Faster Charging Capabilities
Researchers are exploring ways to enable faster charging capabilities, reducing downtime and improving the convenience of EV ownership. This involves advancements in battery chemistry, charging infrastructure, and thermal management systems.
Longer Lifespans
Extending the lifespan of EV batteries is another area of focus, as it can reduce the need for frequent battery replacements and contribute to the overall sustainability of the technology. Improvements in battery management systems, thermal management, and material engineering can help achieve this goal.
Alternative Battery Chemistries and Materials
Researchers are also investigating alternative battery chemistries and materials that could potentially address some of the current limitations and environmental concerns associated with existing technologies. Examples include:
Lithium-sulfur batteries
Lithium-air batteries
Sodium-ion batteries
Solid-state electrolytes
Advanced anode materials (e.g., silicon, tin, and their composites)
These alternative chemistries and materials aim to improve energy density, safety, cost-effectiveness, and sustainability.
Conclusion
As the world transitions towards a more sustainable and eco-friendly transportation future, electric vehicle batteries play a pivotal role. While the current lithium-ion battery technology has enabled the growth of the EV market, ongoing innovations and a focus on sustainability are essential to address the environmental challenges associated with battery production and disposal. By prioritizing recycling, second-life applications, and the transition to renewable energy sources, the EV industry can continue to drive towards a more sustainable and emission-free mobility solution.
FAQs
What is the role of lithium in EV batteries?
Lithium is a highly reactive alkali metal that enables efficient energy storage and release in EV batteries, providing high energy density.
Why is cobalt used in EV batteries?
Cobalt enhances the battery's thermal stability and energy density, although its mining raises environmental and ethical concerns.
What is the function of the anode in a lithium-ion battery?
The anode, typically made of graphite, facilitates the flow of lithium ions during charging and discharging of the battery.
What are the advantages of lithium iron phosphate (LFP) batteries?
LFP batteries offer exceptional thermal stability and safety, although they have a lower energy density compared to other chemistries.
What are solid-state batteries?
Solid-state batteries replace the liquid electrolyte with a solid electrolyte, potentially reducing the risk of thermal runaway and increasing energy density.
Why is battery recycling important for EVs?
Battery recycling helps extend the lifespan of batteries, reduce the demand for new battery production, and promote a circular economy, mitigating the environmental impact of EV batteries.
What is the purpose of a battery management system in EVs?
A battery management system monitors and controls the battery's performance, ensuring optimal operation, safety, and longevity.
What are the potential benefits of silicon anodes in EV batteries?
Silicon anodes have a higher capacity than graphite anodes, enabling higher energy densities and potentially longer driving ranges for EVs.
What are some alternative battery chemistries being explored for EVs?
Researchers are investigating lithium-sulfur, lithium-air, sodium-ion, and other alternative battery chemistries that could potentially improve energy density, safety, cost-effectiveness, and sustainability.
Why is thermal management important for EV batteries?
Proper thermal management is crucial for maintaining battery performance, safety, and lifespan, as excessive heat can degrade the battery and potentially lead to thermal runaway.
Bình luận (0)
Bài viết đề xuất
Pipes and tubing are essential components in various industries, including plumbing, construction, manufacturing, and transportation. They are responsible for carrying liquids, gases, and other materials from one point to another.
Spark plugs play a crucial role in the ignition system of gasoline engines. They are responsible for providing the spark that ignites the air-fuel mixture in the combustion chamber, allowing the engine to run.
A malfunctioning fuel pump can lead to a variety of engine issues, including misfires. The fuel pump is a critical component that delivers pressurized fuel from the tank to the engine's fuel injectors. When it fails, it can cause symptoms that significantly impact the vehicle's performance and drivability. In this article, we'll delve into the relationship between a bad fuel pump and engine misfires, explore the common symptoms of a failing fuel pump, and discuss the steps to diagnose and resolve the problem.
Bài viết liên quan
The automotive industry is at the forefront of a transformative shift towards sustainable transportation solutions. As concerns over carbon emissions and environmental impact continue to mount, innovative technologies like fuel-agnostic engines are emerging as game-changers.
Coal has long been a dominant source of energy worldwide, providing around 37% of global electricity generation. However, the burning of coal is also a major contributor to greenhouse gas emissions, air pollution, and environmental degradation.
Plug-in hybrid electric vehicles (PHEVs) have emerged as a game-changer in the automotive industry, offering a unique blend of electric and gasoline-powered propulsion systems.
Hydrogen fuel cell vehicles (FCVs) represent a promising solution for sustainable mobility, offering zero-emission transportation powered by the chemical energy of hydrogen








